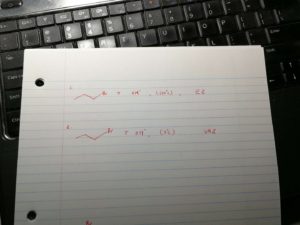
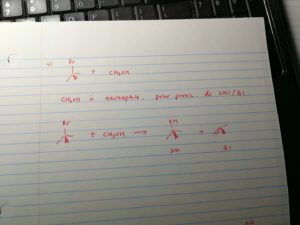I summarize the important points here to help the students to classify the sn1, sn2, e1, e2:
2. for tertiary carbon, when meet strong base, it undergoes e2; if it meet nucleophile, it undergoes sn1 or e1;
3. for secondary carbon, when meet base, it undergoes e2; if it meet nucleopile, depends on the situation.
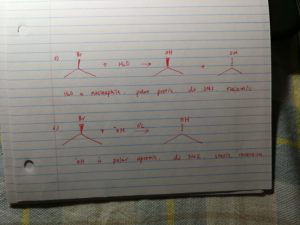
a) if under polar protic nucleopile, it undergoes sn1/e1, it will produce racemic mixture;
b)if under polar aprotic nucleophile, it undergoes sn2, it will produce inversion stereo product.
We summarize all the situation, it will be like the following:
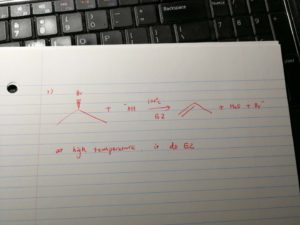
And, at high temperature, elimination has higher priority. if the nuceophile is more hinderance, it will be more likely to undergo elimination.